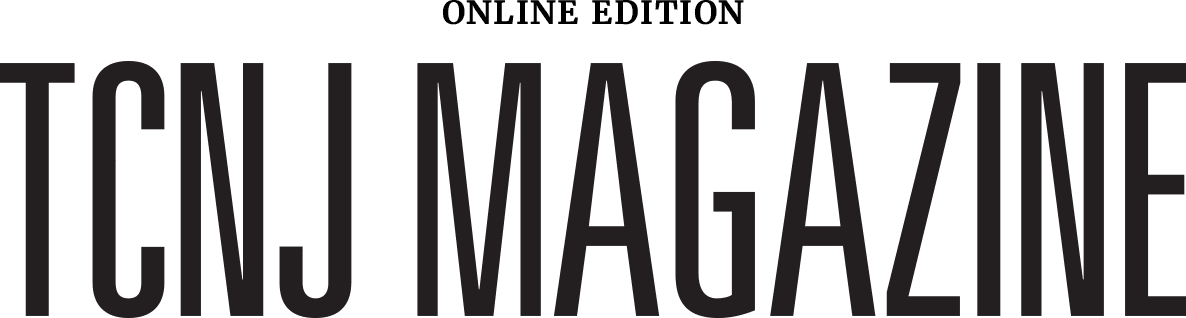Model researcher
Mathematical and computational biologist Jana Gevertz devises equations that mimic tumor progression. Her work is helping in the fight against glioblastoma, a complex and deadly form of brain cancer that poses a persistent challenge to researchers and clinicians.
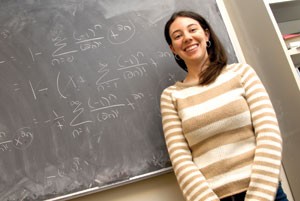
Professor Jana Gevertz’s scholarship has focused on glioblastoma, a complex and deadly form of brain cancer that poses a persistent challenge to researchers and clinicians. Photo (c) Double Exposure Photography
From her earliest days in school, Professor Jana Gevertz was beguiled by the elegance and precision of mathematics. But as her experience widened, she also felt the pull of biology, a powerful, hands-on force in such critical arenas as health care.
“I love how beautifully logical math is. You can’t fight me on a proof—there is no bias. What I love about biology is its ability to impact people and how we live our lives. I’m drawn to the human side of it. So for me, the ideal career was one that used math to benefit people in society,” she says.
Her eureka moment came during her junior year in college when she took the course Differential Equations in Biology and realized “it was possible to work at the interface of math and biology.” That discovery led her to cancer research, where mathematicians, scientists, and clinicians are teaming up to exploit reams of new data—much of it at the molecular level—to advance their understanding of cancer growth and treatments.
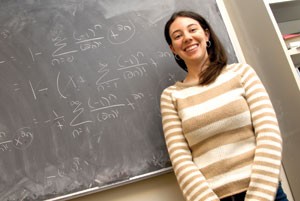
As a specialist in mathematical biology, Gevertz, an assistant professor in the Department of Mathematics and Statistics, devises equations that model the progression of tumors. To date, she has focused on glioblastoma, a complex and deadly form of brain cancer that poses a persistent challenge to researchers and clinicians.
“When I first started researching in this field, mean survival times for these patients hadn’t budged since the 1970s, while we were seeing progress in treating other types of cancer. While tons of data was being gathered about glioblastoma, it was not translating into improved clinical outcomes for patients. So much remained unknown about the disease,” she recalled.
Gevertz was particularly drawn to an aspect of cancer progression that was not well understood: the interactions between different elements of a tumor and the surrounding healthy tissue that lead to its growth. These elements include the genes expressed by cancer cells, the blood vessels that give the tumor oxygen, the stiffness of the surrounding healthy tissue, and immune system responses. While the tumor is initially constrained by its host, it works to reshape its environment so it can thrive.
“Imagine a scenario in which a 17th-century scientist finds a modern-day computer,” she says, by way of analogy. “The scientist would try to understand how each component functions, for instance, the hard drive, the keyboard, and the mouse. But even if the function of each computer part were understood, the scientist would still need to figure out how these pieces interact to produce a functioning computer.
Increasingly, researchers are focusing on pinpointing the interactions that allow the tumor to overcome normal physiological defenses against cancer and coming up with ways to inhibit them. A primary goal of the field, she adds, is to develop a “virtual patient,” a computer program that would use specific information about a patient’s disease to predict how their particular tumor will grow and what treatments would be most effective.
“Patients with the same cancer respond differently. Some people are cured by chemotherapy and some are not. Some people have awful side effects, others none,” she says. “The holy grail of the field is to obtain clinical data on a singular patient, plug that into a computer, and be able to figure out how best to treat them. This is what we call individualized medicine, and it’s something many are striving for in all branches of medicine.”
The relationship between math and biology is not new, Gevertz notes. She points to Gregor Mendel, often called the father of modern genetics, who used comparatively simple math in the 1800s to derive his laws of inheritance, based on breeding experiments with pea plants. The partnership between the two fields has become increasingly productive, however, in recent years.
“There was a boom in the 1990s when biologists began gathering large amounts of data, necessitating a more quantitative approach to their field,” she says. Advances in molecular biology had given them the ability to identify the genes that are mutated in cancer, as well as the proteins expressed by these genes that abet tumor growth. A leap in computing power gave mathematicians powerful new analytical tools.
On occasion, math-based insights can seem counterintuitive, she notes, citing an example in which her equations suggested that using an on/off schedule for a drug that prevents a tumor from growing its own blood supply was more effective than deploying the drug continuously, at full-blast.
“Sometimes you learn things you weren’t expecting to be true,” she says.Supported in graduate school by National Science Foundation and Burroughs Wellcome Fund fellowships, Gevertz comes to her career with a research-intensive background. But she discovered earlier in her academic life that teaching was also important to her.
“What sold me on a career in math ultimately was tutoring. I love working with people and communicating complicated ideas in a way that others can understand,” said Gevertz, who teaches courses ranging from introductory calculus to upper-level classes in applied mathematics. She is thrilled to see her students embracing interdisciplinary subjects as well.
“Students’ interest in fields like mathematical biology is growing rapidly,” she says, noting that her course on the topic next semester is already over-enrolled.
This summer, she will be working on research projects with two of her TCNJ students: a physics major who will study the way cancer cells invade normal tissue and a math major looking at the interactions between a tumor and the body’s immune system. Both students will be using mathematical and computational techniques to tackle challenging biological problems.
“I’m excited that these students are engaging in real scientific research, and also pleased that they will come away from it appreciating that there are multiple ways to approach these important problems,” she says.
Posted on June 5, 2012